Surveillance of Actionable Pulmonary Nodules in Children: The Potential of Thoracic MRI
Karissa A Brazauskas, Jeanne B Ackman and Benjamin Nelson
DOI10.21767/2577-0578.10010
Karissa A Brazauskas1, Jeanne B Ackman2 and Benjamin Nelson1*
1Department of Pediatric Pulmonary Medicine, Massachusetts General Hospital, USA
2Department of Thoracic Radiology, Massachusetts General Hospital, USA
- *Corresponding Author:
- Benjamin Nelson
Department of Pediatric Pulmonary Medicine
275 Cambridge Street, 5th Floor, Boston
MA 02114, USA
Tel: 617-726-8707
Fax: 617-724-2803
E-mail: banelson@mgh.harvard.edu
Received date: November 27, 2015; Accepted date: January 19, 2016; Published date: January 29, 2016
Citation: Brazauskas KA(2015) Surveillance of Actionable Pulmonary Nodules in Children: The Potential of Thoracic MRI. Insights Chest Dis. 1:10.
Abstract
Chest magnetic resonance imaging (MRI) has emerged as a radiation-free alternative to computed tomography (CT) for the evaluation of thoracic abnormalities, including pulmonary nodules; particularly those measuring one centimeter or greater, the size for which magnetic resonance has demonstrated 100% sensitivity. We describe the case of a 9-year-old girl presenting with necrobiotic pulmonary nodules secondary to Crohn’s disease in which MRI was successfully utilized to monitor treatment response. This case demonstrates that chest MRI may be an effective and practical imaging modality that can be used for the surveillance of actionable pulmonary nodules in children.
Keywords
Pulmonology; Pediatrics-radiography; Pulmonary nodules; Magnetic resonance imaging (MRI); Radiation
Introduction
Computed tomography (CT) exposes patients to ionizing radiation which is of particular concern for children given the potential increased lifetime cancer risk [1], especially those requiring interval follow-up studies; yet, it remains the gold standard in chest imaging. Advances in chest magnetic resonance imaging (MRI) techniques have allowed for improved visualization and evaluation of the lungs and airways in children [2]. Consequently, chest MRI has emerged as an attractive, radiation-free alternative to CT for the evaluation and follow-up of thoracic abnormalities.
The diagnostic evaluation of pulmonary nodules is challenging. There are no evidence-based guidelines to direct management in children. MRI can reliably identify pulmonary nodules 5mm or greater in both children and adults [3-5], but whether MRI can be used to follow the progression of pulmonary nodules or their response to treatment has not been described. We report a case in which MRI was employed for surveillance of treatment response in a pediatric patient with pulmonary nodules secondary to Crohn’s disease.
Case Report
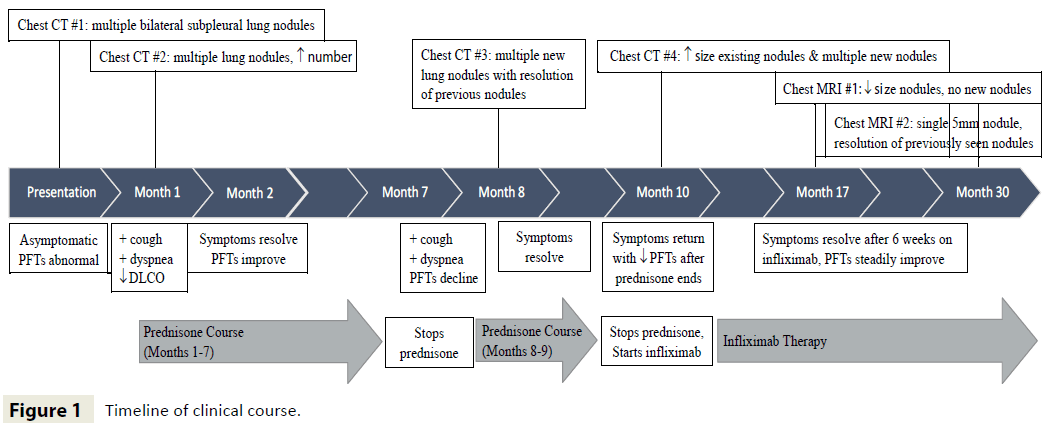
A 9-year-old girl with Crohn’s disease hospitalized for Clostridium difficile colitis was incidentally found to have multiple bilateral pulmonary nodules discovered on a contrast-enhanced CT of the abdomen. At that time, she had no respiratory symptoms and her pulmonary examination was unremarkable. However, pulmonary function tests (PFTs) revealed a restrictive pattern and low carbon monoxide diffusing capacity (DLCO), which were decreased from normal PFT results obtained nine months earlier in the setting of a viral illness (see Table 1 for all PFT values). A dedicated chest CT with intravenous contrast revealed bilateral upper and lower lobe pulmonary nodules in a primarily subpleural distribution, measuring up to 13 mm in greatest dimension. Margins were ill-defined and some exhibited central hypoattenuation, a feature consistent with central necrosis. Infectious etiologies seemed unlikely given the lack of overt respiratory symptoms. There was no evidence of a primary neoplasm making metastatic disease unlikely. Rheumatologic conditions such as granulomatosis with polyangiitis were ruled out by a negative cANCA, absence of renal disease and a declining ESR. Therefore, further workup of the pulmonary nodules was deferred until her GI symptoms resolved (Figure 1).
| Timeline | FEV 1 | FVC | TLC | DLCO | ||||
|---|---|---|---|---|---|---|---|---|
| L | %pred | L | %pred | L | %pred | mL/min/mmHg | %pred | |
| Baseline | 1.74 | 84 | 1.83 | 78 | 2.77 | 94 | 15.39 | 92 |
| Admission | 1.54 | 67 | 1.59 | 61 | 2.48 | 75 | 11.79 | 65 |
| 2 weeks later | 1.57 | 68 | 1.57 | 60 | 2.46 | 74 | 10.36 | 57 |
| 1 month OP | 1.82 | 79 | 1.93 | 73 | 2.65 | 80 | 13.96 | 77 |
| 1 month AP | 1.77 | 71 | 1.99 | 70 | 2.65 | 73 | 12.14 | 62 |
| 6 weeks OI | 1.73 | 67 | 2.03 | 69 | 2.77 | 73 | 14.63 | 72 |
| 12 months OI | 2.44 | 84 | 2.68 | 79 | 2.97 | 67 | 18.08 | 79 |
PTA: Prior to admission; OP: On prednisone; AP: After prednisone; OI: On infliximab; FEV 1: Forced expiratory volume in 1 second; TLC: Total lung capacity; DLCO: Diffusing capacity of the lung for carbon monoxide.
Table 1: Timeline of pulmonary function testing.
Two weeks after her abdominal symptoms resolved, she developed cough, pleuritic chest pain, and exertional dyspnea. Her chest was clear on auscultation and she was not clubbed. Repeat PFTs showed persistent restriction and declining DLCO. In preparation for lung biopsy, a second chest CT was obtained one month after the initial scan (Figure 2). Several of the nodules had increased in size while others had decreased.
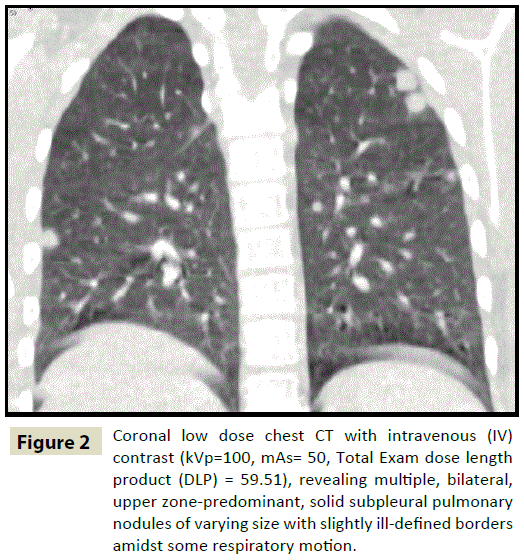
Figure 2: Coronal low dose chest CT with intravenous (IV) contrast (kVp=100, mAs= 50, Total Exam dose length product (DLP) = 59.51), revealing multiple, bilateral, upper zone-predominant, solid subpleural pulmonary nodules of varying size with slightly ill-defined borders amidst some respiratory motion.
A thoracoscopic lung biopsy contained a single subpleural lung nodule, measuring 1 cm in diameter. The nodule had a central focus of necrobiosis surrounded by spindle-shaped histiocytes in a parallel array, consistent with a necrobiotic nodule. Necrobiotic nodules have been described as an extraintestinal manifestation of Crohn’s disease [6].
Treatment was initiated with 40 milligrams (mg) of prednisone once daily for four weeks followed by a five month taper. Symptoms resolved and PFTs approached baseline one month into therapy. Unfortunately, within two weeks of discontinuing prednisone, she developed recurrent respiratory symptoms and pulmonary function declined. A third chest CT, obtained to evaluate radiographic disease burden seven months from the previous scan, revealed near resolution of previously noted nodules; however, new nodules had developed bilaterally in the upper lobes, the largest measuring 19 mm. Another prednisone course (40 mg daily x 4 weeks), including a one month taper, resulted in transient improvement; however, dyspnea and chest pain recurred several weeks after steroids were discontinued. A chest x-ray revealed multiple nodules, but was insufficient to accurately delineate changes in nodule size and number. Since alternative immune-modulatory therapies were being considered, a fourth chest CT was deemed necessary, two months after the prior one, in order to establish a pre-treatment baseline. Existing nodules had grown larger, with the appearance of new nodules in the right lower lobe (Figure 3A). The decision was therefore made to initiate infliximab therapy (5 mg/kg every 8 weeks). Six weeks later, her symptoms had resolved and PFTs were improved.
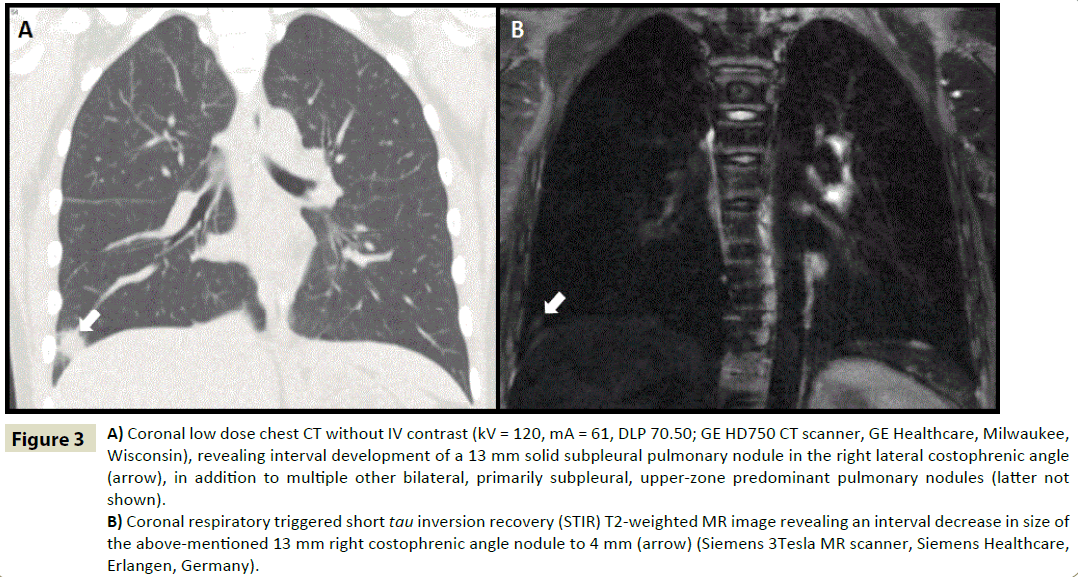
Figure 3: A) Coronal low dose chest CT without IV contrast (kV = 120, mA = 61, DLP 70.50; GE HD750 CT scanner, GE Healthcare, Milwaukee, Wisconsin), revealing interval development of a 13 mm solid subpleural pulmonary nodule in the right lateral costophrenic angle (arrow), in addition to multiple other bilateral, primarily subpleural, upper-zone predominant pulmonary nodules (latter not shown).
B) Coronal respiratory triggered short tau inversion recovery (STIR) T2-weighted MR image revealing an interval decrease in size of the above-mentioned 13 mm right costophrenic angle nodule to 4 mm (arrow) (Siemens 3Tesla MR scanner, Siemens Healthcare, Erlangen, Germany).
To ensure resolution of the pulmonary nodules, additional imaging was necessary. To avoid further ionizing radiation, a chest MRI was obtained six months after initiation of infliximab (Figure 3B), revealing decreased size of previously seen nodules, resolution of other nodules, and no new nodules. This MRI was obtained on a Siemens 3T magnet (Siemens Healthcare, Erlangen, Germany) and was performed with cardiac-gated steady state free precession images (True FISP), respiratory triggered 3-dimensional (3D) short tau inversion recovery (SPACE) T2, cardiac-gated, respiratory triggered short tau inversion recovery (STIR) T2, and breath-hold 3D ultrafast gradient echo T1-weighted images (VIBE) without and with fat saturation. A follow-up MRI performed one year later with a similar protocol revealed a single 5 mm nodule in the right lower lobe, with resolution of previously identified nodules. The MRI findings correlated with the patient’s clinical picture, as she remained asymptomatic.
Discussion
Pulmonary manifestations of Crohn’s disease include large and small airway disease, interstitial disease, and necrobiotic nodules. There are only a few cases reported in the literature describing necrobiotic lung nodules in patients with Crohn’s disease, although respiratory involvement as a whole, particularly subclinical lung dysfunction is generally accepted [6]. Diagnosis of necrobiotic nodules includes CT scan and lung biopsy. The mainstay of therapy is corticosteroids, but some patients may require immunomodulatory agents such as infliximab [7]. Currently, serial CT scans are utilized to follow response to therapy.
A recent study demonstrated that rapid lung MRI has a high correlation, sensitivity, and specificity compared to CT scan in the detection of pulmonary nodules in children with leukemia and persistent febrile neutropenia [8]. Our case suggests it may also be possible to use MRI to follow response to treatment, avoiding the need for repeated radiation.
Initially, we hoped to use clinical symptoms and PFTs as an indirect measure of the patient’s lung disease; however, this proved inadequate as the nodules grew despite the absence of overt clinical symptoms. Our patient had undergone six CT scans within a two-year period so we needed an imaging modality to follow disease burden without exposure to continued radiation.
Multiple studies have demonstrated that MRI can reliably detect lung nodules larger than 5 mm [3,4,9], with various MR pulse sequences studied and proposed for their detection including single shot fast spin echo T2-weighted imaging, steady state free precession imaging, short tau inversion recovery imaging, and 3-dimensional ultrafast gradient echo imaging without and with intravenous contrast.
The primary advantage of MRI is its ability to detect actionable nodules without ionizing radiation. Disadvantages include higher cost, longer scanning time, operator dependency, need for a cooperative patient who can breath-hold and lie still for 15 to 45 minutes, and lower spatial resolution compared to CT. With continued improvements in MR hardware and software, many of these disadvantages are diminishing.
The first MRI in this case was obtained six months after the initiation of infliximab. From a proof of concept standpoint, it would have been ideal to have performed a baseline MRI prior to treatment; however, the clinical reality was otherwise. The MRI did reveal significant improvement compared to the prior CT scan as the nodule in the right lower lobe decreased substantially in size from 13 to 4 mm (Figure 3A and 3B); and this radiographic finding correlated with the patient’s clinical symptoms and PFTs. We are confident about the fact that we did not miss any nodules larger than 5 mm based on proven capability of MR in this regard [3-5].
This case suggests that thoracic MRI has the potential to monitor the progression of actionable pulmonary nodules and their response to treatment, while averting the need for ionizing radiation. Prospective studies need to be performed to establish the exact role of thoracic MRI, but we believe this effective, radiation-free option will be invaluable for the pediatric population and, in particular, for patients requiring recurrent surveillance imaging.
Conflicts of Interest
None
References
- Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. American journal of roentgenology 176:289-296.
- Puderbach M, Eichinger M (2010) The role of advanced imaging techniques in cystic fibrosis follow-up: is there a place for MRI? Pediatric Radiology 40: 844-849.
- Kurihara Y, Matsuoka S, Yamashiro T, Fujikawa A, Matsushita S, et al. (2014) MRI of pulmonary nodules. American journal of roentgenology 202: W210-216.
- Gorkem SB, Coskun A, Yikilmaz A, Zurakowski D, Mulkern RV, et al. (2013) Evaluation of pediatric thoracic disorders: comparison of unenhanced fast-imaging-sequence 1.5-T MRI and contrast-enhanced MDCT. American journal of roentgenology 200: 1352-1357.
- Biederer J, Hintze C, Fabel M (2008) MRI of pulmonary nodules: technique and diagnostic value. Cancer Imaging 8: 125?130.
- Warwick G, Leecy T, Silverstone E, Rainer S, Feller R, et al. (2009) Pulmonary necrobiotic nodules: a rare extraintestinal manifestation of Crohn?s disease. European Respiratory Review 18: 47-50.
- Krishnan S, Banquet A, Newman L, Katta U, Patil A, et al. (2006). Lung lesions in children with Crohn's disease presenting as nonresolving pneumonias and response to infliximab therapy. Pediatrics 117:1440-1443.
- Sodhi KS, Khandelwal N, Saxena KA, Bhatia A, Bansal D, et al. (2016) Rapid lung MRI-paradigm shift in evaluation of febrile neutropenia in children with leukemia: a pilot study. Leukemia and Lymphoma 57:70-75.
- Baez JC, Ciet P, Mulkern R, Seethamraju RT, Lee EY (2015) Pediatric Chest MR Imaging: Lung and Airways. Magnetic Resonance Imaging Clinics of North America 23: 337-349.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences